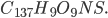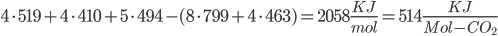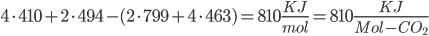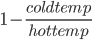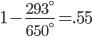Photovoltaic solar cells. Solar PV.
This is not an easy thing to describe. For some, you may want to just skip past the technical section, cause it is pretty technical.
Solar PV: they used to take as much electricity to make as they produced in their lifetime. Now they they produce about 5x as much energy as they take to make, and the time to break-even on emissions compared to our cleanest fossil fuel stations is about 6 years (see Kannan, 2005, Lifecycle Assessment Study of Solar PV). Of all the clean technologies (nuclear power excluded), this is the only one with the potential to supply world energy needs (that is the subject of a later post).
In other words, when you hear some fool saying that solar panels take as much energy to manufacture as they ever produce, they are referring to a specific type of solar cells called thin film. A type that was made in the 70s and 80s and only goes into things like calculators. Feel free to ask them to stop being foolish.
Some Math
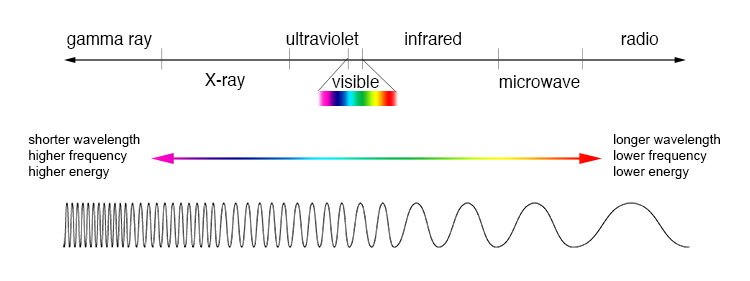
The light we see is not a homogenous single color. In fact, the light we see is not even all the light that is coming from the sun. Infrared and UV rays are also light, but we cannot see them at all. All this light is just an electromagnetic wave. The waves have different wavelengths, but the same speed, and so all the different wavelengths travel together. What we see is a blend of a tiny part of the electromagnetic spectrum.
The amount of energy contained in a photon is equal to
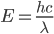 where
where 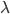 is the wavelength. h and c are Planck's constant and the speed of light, respectively.
is the wavelength. h and c are Planck's constant and the speed of light, respectively.
Smaller (shorter) wavelengths give more energy. This is easily shown just by plugging a smaller number into the denominator. Stuff in the infrared is long wavelength, and stuff in UV, X-ray, Gamma ray, etc, are really short wavelength.
Technical Stuff
There is no easy way to do this. I am going to use some terminology that most of you all are unfamiliar with.
A Photo Voltaic (PV) solar panel is a sandwich of two materials. The materials are largely the same, with a few key differences. Both are likely made of silicon (processed sand). But each one has very specific impurities put into them, in a process called doping (not the same type that Lance Armstrong does). This doping is incredibly technical, and very skilled chemists are paid a ton of money to figure out how to do it.
Doping
I won't get into specific materials. Some elements cause there to be a shortage of electrons, or a electron hole, in the whole material (p-type semiconductor). Other elements cause an excess of electrons (n-type semiconductor). So you have one material that can accept electrons, and another material that can give electrons. Putting them together (literally stacking them together) makes magic happen. And by magic, I mean quantum mechanics. Which to most people, including many who study it, is no different than magic.
Just because one has more electrons doesn't mean it wants to be nice and share them. The electron in the n-type literally needs to be excited to be shared. And in PVs, what turns the n-type material on is sunlight. More specifically, photons. Photons are particles of light. ("But Jason!" you say, "Isn't light, like, a wave?" to which I say, "It is both a wave and a particle! Please don't ask me why, just accept it.") Photons contain energy based on their wavelength. Shorter wavelength, more energy (see above).
Here's the fun part. It takes energy to make the n-type semiconductor want to party with the p-type semiconductor. There is a threshold level of energy that needs to be met to kick that electron up from the n-type to the p-type. Too little energy, and the photon doesn't get excited enough to go to the electricity-production party. If there is enough energy from the particular wavelength of light to make that electron jump, then is does jump.
But what if there is more than enough? This threshold level is pretty much determined. Any extra energy will be wasted. This is why PV cells are not particularly efficient. There is a huge amount of light that is too low-energy (all of infrared) for the cell to gather any energy from. There is a lot of light that has much higher energy than required to meet the threshold energy as well. The excess energy is wasted as heat. This can be solved by having a multiple junction cell (multiple junctions just means it has a bunch of different width absorption gaps, so it can harness tons of different energy levels in light). It is capable of absorbing more wavelengths of light, increasing efficiency. And since it has multiple junctions, it is also more expensive and complicated to produce.
Hokay, so, what happens next? You have an electron that has jumped the gap. Then you close the circuit by connecting them with a wire. The electron will go home, back to the n-type, and create an electric charge on its way down. That's about it.
New Methods
Transistors are expensive. Normal glass optics are relatively cheap. The transistors absorb about 20% of the light that hits them. So it would make sense to use cheap optics to focus more light on the transistors and make the transistors small, yes?
One problem with these cells is how warm they get. If they warm up too much, they begin to lose efficiency. Here we see that we have a tradeoff. We want more optics and fewer cells, but if we do this, they get too warm. They stop being efficient. Some scientists and engineers are working on increasing the efficiency of the cells instead, to get more electricity from light. Otherwise, there are clever ways that some mechanical engineers are trying to get around these issues. One new design uses focusing mirrors and liquid cooling to get around this issue.
The pertinent stuff
Everything pertinent, like insolation and weather, was in the last solar article.
Next post: rounding up some of the stray power sources: tidal, geothermal, wave, and then I am pretty much done.
Thanks for reading.
-Jason Munster