Power Plants! Primary Energy vs. Electricity!
Energy and electricity are the backbone of our civilization and economy, right? (Hint: the answer is yes) How do we get all this electricity we got kickin’ around? That’s a pretty complicated topic. This is gonna take a few posts, but we’ll get there. Today is energy and electricity, and how they relate. Later will be a more detailed look at some power plants. I don't think most people will want to skip the math section this time, as it is both interesting and kind of fun.
First, The Math!
The great bulk of our electricity generation amounts to complicated ways to boil water. Even nuclear plants generate electricity by harvesting the heat of a nuclear reaction to boil water. In all these power plants, application of heat turns that water into steam. That water to steam thing expands the water a whole lot and creates positive pressure (and thus wind) to drive a turbine. Driving a turbine spins a coil of metal in a magnet, converting mechanical energy into electric energy.
Picture!
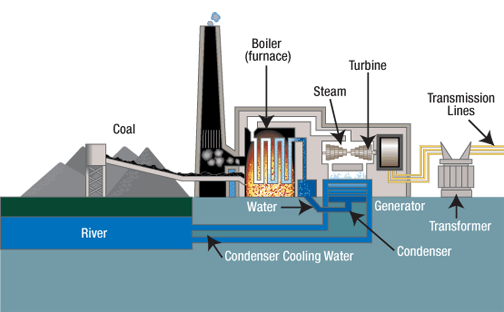
Block diagram of a coal / thermal / steam / fossil fuel power plant. Source is TVA website (government authority that built powerplants in a huge chunk of the south)
Nuclear plants, coal plants, and natural gas plants all produce electricity via boiling water. For this reason, these powerplants are called thermal plants. Thermal --as in heat. Right? Cool.
Hokay, so how efficient are these thermal plants? The maximum efficiency of a heat engine (which a thermal plant is) is 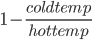 (temperature in Kelvin!). Kelvin is the celcius scale, but is always positive. Absolute zero is 0 kelvin. 273.15°K is 0°C. Using Kelvin makes it way easier to do science.
(temperature in Kelvin!). Kelvin is the celcius scale, but is always positive. Absolute zero is 0 kelvin. 273.15°K is 0°C. Using Kelvin makes it way easier to do science.
The hot temperature is the temperature of our steam that drives the turbine. The cold temperature comes from whatever cold water they have handy. Often it is seawater, a lake, or a river. The cold water will probably be around 20C, or 293K. The hot temperature is the temperature of the steam in the thermal plant. Lets say that is 650K (roughly 377 celcius, quite a bit above the boiling temperature of water at 100C! This is very hot steam!)
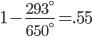 or 55% efficient.
or 55% efficient.
This is the maximum theoretical efficiency of a thermal power plant. It is how much heat can be converted to electricity. So say that our gallon of gasoline with 33kwh of power was burnt in a perfectly efficient power plant with 650°K steam. We would only obtain 33*.55 or roughly 18kwh of our 33kwh. The other 15kwh will just become waste heat. This maximum efficiency is never attained. There are always extra heat losses and efficiency losses throughout the power plant. Many coal-fired power plants only achieve 35% efficiency. The same gallon of gas would actually only be able to produce closer to 12kwh of electricity from our 33kwh of energy in the gallon of gasoline.
More math! (only slightly related, cause I want to do more math). Calculating how much heat is produced from stopping a bicycle is a fun way to get an idea of how heat and motion relate. For the setup, assume that I weigh 90kg and am bicycling at 10 meters per second.
The amount of kinetic energy (the energy just from moving) that I have is .5*mass*velocity2
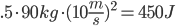 . This is all of .125 watt hours. Not much at all. All of this kinetic energy is converted to heat energy in the brakes. Compare this to the energy from the first post about heating up a cup of tea, which took 31kj, or nearly 100 times as much. How bout a car? It travels at about 30m/s on the highway (about 70mph). It weighs 1000kg.
. This is all of .125 watt hours. Not much at all. All of this kinetic energy is converted to heat energy in the brakes. Compare this to the energy from the first post about heating up a cup of tea, which took 31kj, or nearly 100 times as much. How bout a car? It travels at about 30m/s on the highway (about 70mph). It weighs 1000kg.
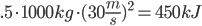 , or 125 watt hours. Stopping this car requires dissipation of a lot of heat at the brakes! That’s like 15 cups of tea worth of heat. Another way to look at this is that stopping this car would provide only enough energy to power a 100 watt lightbulb for an hour and 15 minutes. So that is how much energy the car has in its motion. It takes a lot more energy to get the car going that fast, though. Why? Cause the engine in our car is also a heat engine. It’s not very efficient at converting energy. A car is about 25% efficient. The other 75% is all converted to waste heat. The energy it takes to accelerate your car to 70mph could power a 100 watt incandescent lightbulb for 5 hours.
, or 125 watt hours. Stopping this car requires dissipation of a lot of heat at the brakes! That’s like 15 cups of tea worth of heat. Another way to look at this is that stopping this car would provide only enough energy to power a 100 watt lightbulb for an hour and 15 minutes. So that is how much energy the car has in its motion. It takes a lot more energy to get the car going that fast, though. Why? Cause the engine in our car is also a heat engine. It’s not very efficient at converting energy. A car is about 25% efficient. The other 75% is all converted to waste heat. The energy it takes to accelerate your car to 70mph could power a 100 watt incandescent lightbulb for 5 hours.
Okay! Back to the big picture!
Electricity Generation and Primary Energy
What is primary energy? Let’s step back and figure out all the way we use power plants and fossil fuels to make our lives easier. The first one is obvious: electricity. It powers our houses, lights, electronics, etc., and sometimes even is used for heating our houses. And it is definitely used to cool our houses with an air conditioner. The next obvious one is your car, which doesn’t run on electricity. We pour gasoline in, and then the engine converts the gasoline to kinetic energy and the car moves. After electricity production and transportation, what we have left is heating for home and industrial purposes. This can be done with coal (many poorer countries use low-quality coal to cook with), wood, natural gas, or any other combustible. As discussed above, electricity is produced by burning fossil fuels to produce heat. Electricity is a byproduct of primary energy.
So that is what primary energy is. All that stuff added together. When most people think “Energy” they equate it with electricity. Now you know differently! A great example of this error was the reporting on the Fukushima nuclear meltdown in Japan. During the reporting, news organizations would regularly say that 20% of energy supplied in the US is from nuclear power. This, however, is wrong! Only about 10% of energy supplied in the US is nuclear. The 20% number is how much of our electricity comes from nuclear. First, what causes these two numbers to be different? And second, why do we even care?
That difference in between resource inputs and electricity output is part of the difference between primary energy and electricity. If we live in a tiny country that uses only one power plant to provide all of its electricity needs, and it uses only electricity for everything. It burns 100 gallons of gasoline an hour (this would never be a power plant input, but let’s keep it simple with numbers we know). It would burn 3340kwh of energy in that hour, and produce 1200kwh of electricity in that hour. Since this is our only energy use in the entire country, the former is primary energy use (3340kwh), and the latter is electricity use (1200kwh)!
This pretty picture from the DOE pretty much explains it all. And now you can explain it to others!
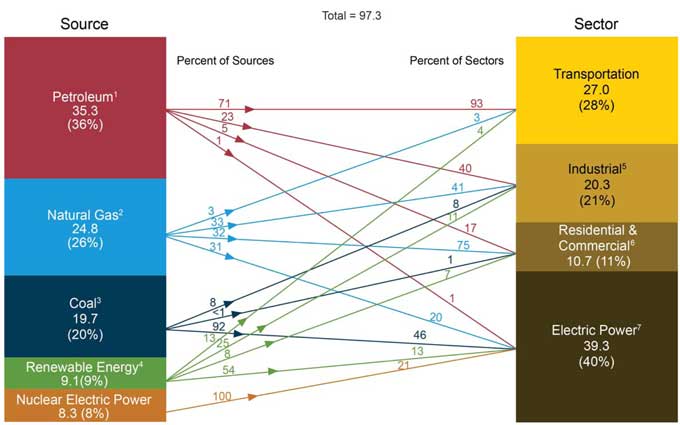
Primary Power is all the things that produce energy, whether it is burning coal in a power plant or gasoline in your car. These are our sources of energy, and where they end up.
What about power plants that don’t use fossil fuels, like hydroelectricity? Easy! A 10MW hydro plant run for an hour registers as 10MWh both primary energy use and electricity use. An important takeaway here: if we lived in a 100% renewable and 100% efficient economy, primary energy use could nearly equal electricity use.
So this is one reason we care about the difference between primary energy use and electricity use. It is a measure of how efficiently we are using our resources, and how much we are getting for all that CO2 we are shoving into the air when we make electricity.
Let’s go back to everything that is primary energy. We have the inputs of heating, cars, and electricity production. Cars and heat are entirely primary energy use. We don’t really use electricity in many places to run either. So now we have everything we need to know to analyze primary energy vs. electricity, efficiencies of power plants, and energy efficiencies of whole economies! Pretty sweet, eh?
Okay, we are pretty much done here. I want to mention one last thing. That small powerplant we had, the one that burned 100 gallons of oil in an hour to produce 1200kwh? A real powerplant in the US produces in megawatts. Like 600 to 1000 MW. 600 times the size of our small powerplant. Even a small 600MW powerplant one would require 60,000 gallons of oil per hour. This is the scale we are talking when we have power plants.
-Jason Munster

Pingback: Coal Power Plants | Jason Munster's Energy and Environment Blog
Pingback: Natural Gas Power Plants | Jason Munster's Energy and Environment Blog
Pingback: Nuclear Power Plants! | Jason Munster's Energy and Environment Blog
Pingback: Wind Power | Jason Munster's Energy and Environment Blog
Pingback: Solar Power | Jason Munster's Energy and Environment Blog
Pingback: Power Grid | Jason Munster's Energy and Environment Blog